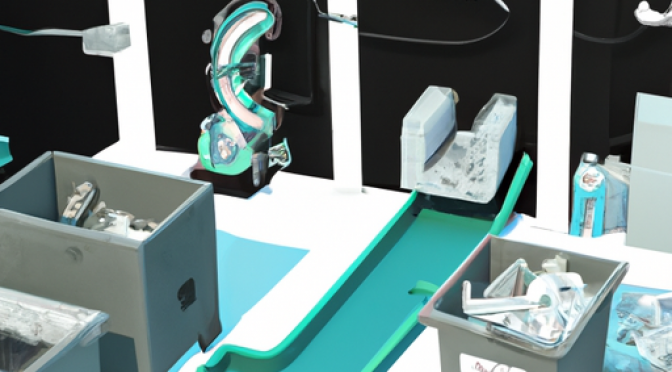
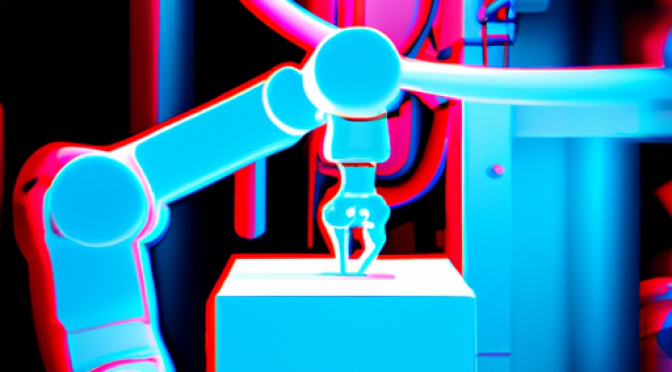
Introduction
Machine learning algorithms have revolutionized various industries, and manufacturing is no exception. In today’s dynamic market, where product demands are constantly changing, adaptive manufacturing setups are crucial for businesses to stay competitive. This article explores how machine learning algorithms …